Robustness of aerosol delivery of amikacin liposome inhalation suspension using the eFlow® Technology - ScienceDirect

Robustness of aerosol delivery of amikacin liposome inhalation suspension using the eFlow® Technology - ScienceDirect
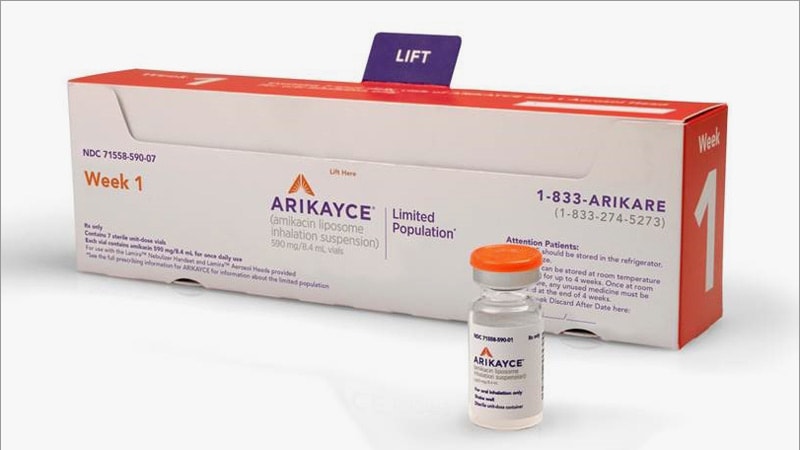
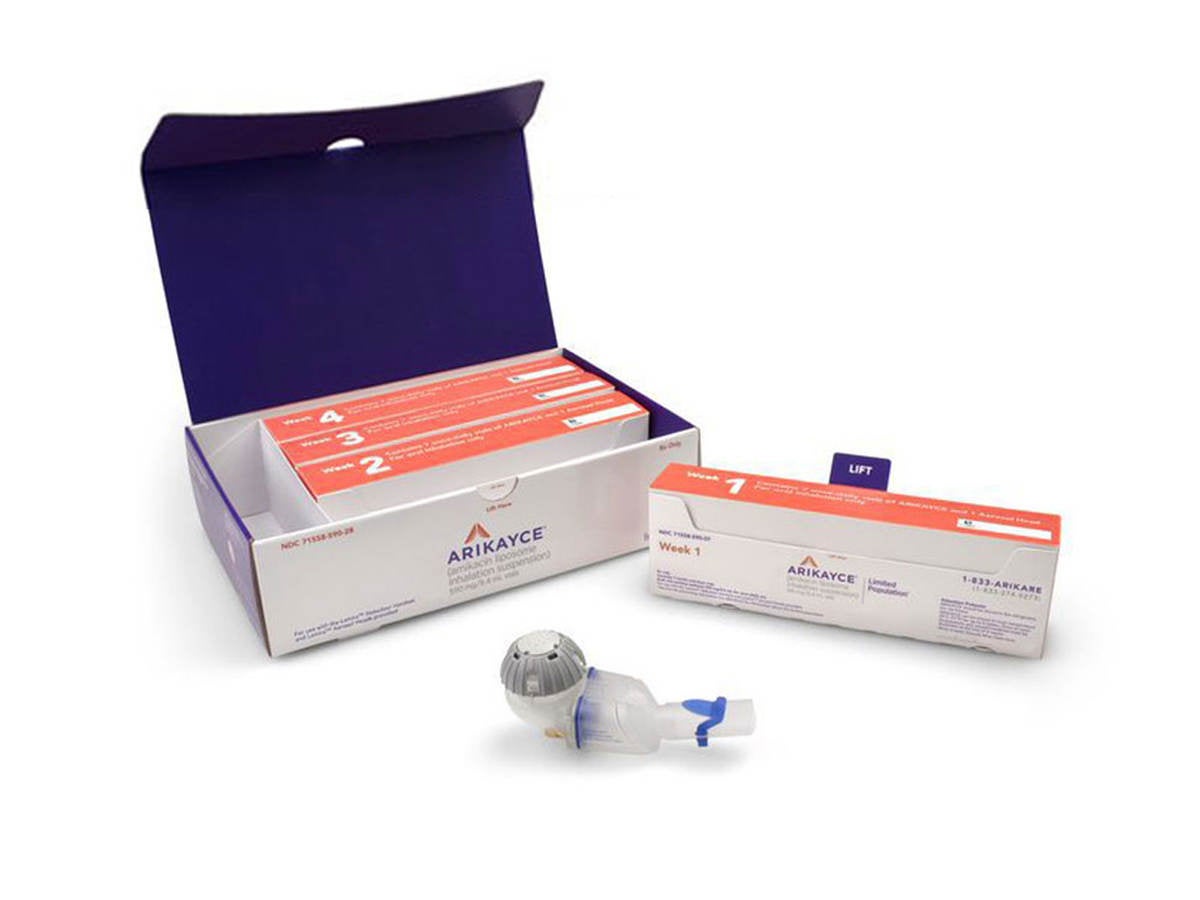
ARIKAYCE® (amikacin liposome inhalation suspension) is the first and only FDA-approved therapy indicated for the treatment of
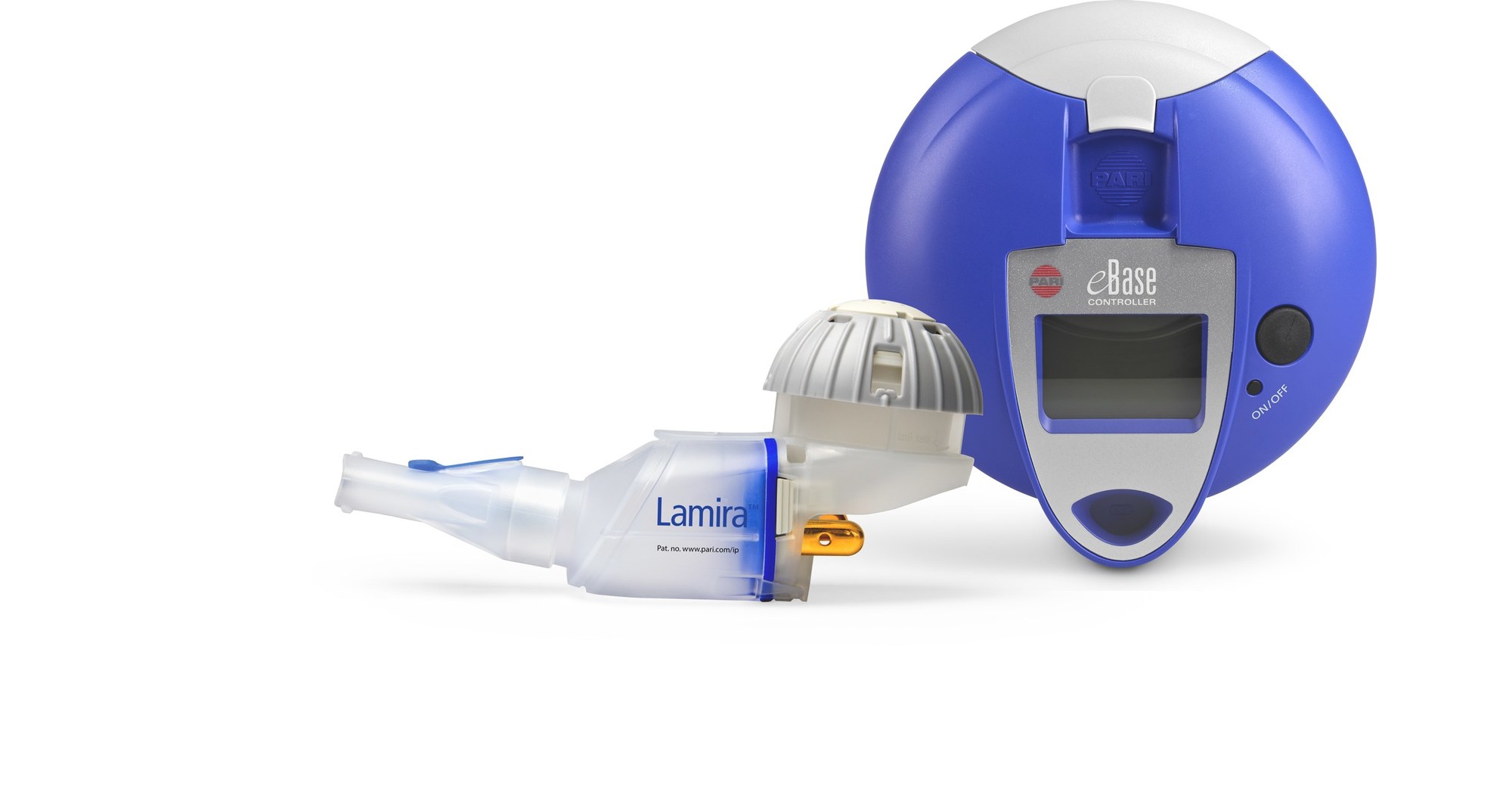
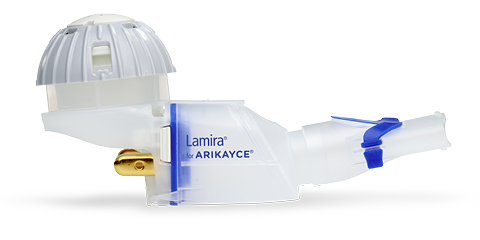
PARI Pharma's eFlow® Technology device, LAMIRA™, approved as the only nebulizer system to deliver Insmed's ARIKAYCE® (amikacin liposome inhalation suspension)

Market Authorizations: ARIKAYCE, clonoSEQ Assay, XOFLUZA – Drug and Device Digest - DRUG AND DEVICE DIGEST